1 Which of the Following Best Describes an Atom
B They are both phosphorus cations. Atom is the basic unit of matter.
A a space in an atom where an electron is most likely to be found b space where electrons are unlikely to be found in an atom.

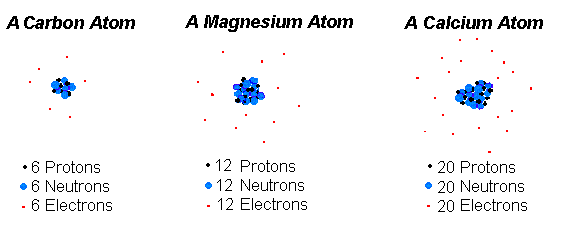
. Which of the following best describes an isotope. Benchmark 1 Review DRAFT. 100 1 rating Mg is the atom whose atomic number is 12.
E They each contain 1 neutron. One carbon atom forms a double bond with an oxygen atom and two single bonds with two hydrogen atoms. They can do so by achie.
Atom 1 Atom 2 31 15 P 32 15 P A They contain 31 and 32 electrons respectively. 16_ 16 Which of the following best describes how an atom with atomic number 12 would behave in terms of forming bonds with. Nucleus with 7 protons and 8 neutrons surrounded by 7 electrons Atom 2.
Which of the following BEST describe the quantum mechanical model of an atom. An atom that has bonded with another atom to form a molecule. SPS1a DOK 1 O protons and electrons grouped together in a random pattern O protons and electrons grouped together in an alternating pattern a core of protons and neutrons surrounded by electrons O a core of electrons and neutrons surrounded by protons.
One carbon atom forms a triple bond with another carbon atom and a double bond with an oxygen atom. Polar covalent bond c. Which statement about subatomic particles is NOT true.
Which of the following statement best describes an 1Which of the following statement best describes an atom. 85A Which of the following best describes an electron. It describes the electrons that move in definite orbits around the.
One carbon atom forms five single bonds with five hydrogen atoms. A Structurally variant atoms which always have a mass number of 1. It describes the electron probability distribution that determines the most likely location of an electron C.
20 Questions Show answers. View the full answer. A single space within an atom that contains all electrons of that atom.
One carbon atom forms a quadruple bond with another carbon atom. 1 Mg atom and 1 Cl2 molecule b. 1 positive Mg ion and 2 negative Cl ions d.
Pure covalent bond b. Which of the following best describes an atom. An atom that has gained or lost electrons and has an electrical charge.
An isotope is an atom of an element that varies in mass number due to variation in the number of. A Electronegativity is what happens when an atom gains an electron to become an anion. Which of the following best describes the relationship between the atoms described below.
1 MgCl2 molecule c. Which of the following best describes an orbital. Which statement best describes the part of the atom that is shown by the arrow.
C They are isotopes. The attraction that one atom has for another atom. A nucleus that is mostly empty space with charged particles circling around it.
D They are both isotopes of phosphorus. Which of the following best describes the structure of an atom. An atom that has more or fewer neutrons than protons.
2Which subatomic particles is electrically neutral. C Electronegativity is the energy lost when an atom gains an electron. Is it C 2 A covalent bond is.
Any atom in the periodic table wants to be stable. CLICK HERE to Calculate Price and Order An Original SOLUTION to This Assignment. Chemistry questions and answers.
Nucleus with 7 protons and 7 neutrons surrounded by 7 electrons Both atoms have the same atomic number but different atomic mass numbers. C They are both phosphorus anions. Proton 3Which among the following.
Atom 1 Atom 2 1 3 H H 1 1 A They are isomers. Which one of the following statements best describes electronegativity in atoms. A positively charged nucleus circled by widely spaced negatively charged particles.
B Structurally variant atoms which have the same number of neutrons and protons but differ in the number of electrons they contain. It has no charge and about the same mass as a proton. D They contain 1 and 3 protons respectively.
We review their content and use your feedback to keep the quality high. B Electronegativity is the attraction an elements nucleus has for the electrons in a chemical bond. Which of the following best describes best describes an atom.
E They contain 31 and 32 protons respectively. 3 Show answers Another question on Chemistry. Atom is made up of.
Which one of the following best describes the simplest unit of MgCl2. A neutral nucleus circled by particles with positive and negative charges. Which of the following best describes an isotope.
1 Mg atom and 2 Cl atoms e. It has a negative charge and much less mass than a proton. 15 Which of the following best describes the relationship between the atoms described below.
It has a positive charge and much more mass than a neutron. It describes that an atom had a small dense positively charged center called the madeus. An atom that has changed into another atom through loss or gain of protons.
Which of the following best describes the type of bond that would form between a carbon atom and an oxygen atom and why. 8th - University grade.

Atomic Number Mass Number And Isotopes Mass Number Atomic Number Atom

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets
0 Response to "1 Which of the Following Best Describes an Atom"
Post a Comment